
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
Shijiazhuang Dingmin pharmaceutical Sciences Co.,Ltd
sales@dingminpharma.com
86-311-67591193
![]() December 20, 2019
December 20, 2019
First imitation
On September 29, 2019, Zhengda Tianqing Pharmaceutical Group first approved azacitidine for injection, with the trade name: Weishou. It was issued three times before and was finally approved for marketing. It was deemed to have passed the consistency evaluation of generic drugs.
This product is suitable for the treatment of the following adult patients: Intermediate--2 and High-Risk Myelodysplastic Syndrome (MDS) in the International Prognostic Scoring System (IPSS); Chronic Myelomonocytic Leukemia (CMML); ) Classification of acute myeloid leukemia (AML), bone marrow blasts is 20% to 30% with multiple lineage abnormalities.
Azazacitidine is a cytosine nucleoside analog. It is a demethylated drug with dual action mechanisms and currently the only clinical study that has proven to improve overall survival (OS). It has a clear mechanism of action, positive efficacy, and safety. High sex and wide clinical application.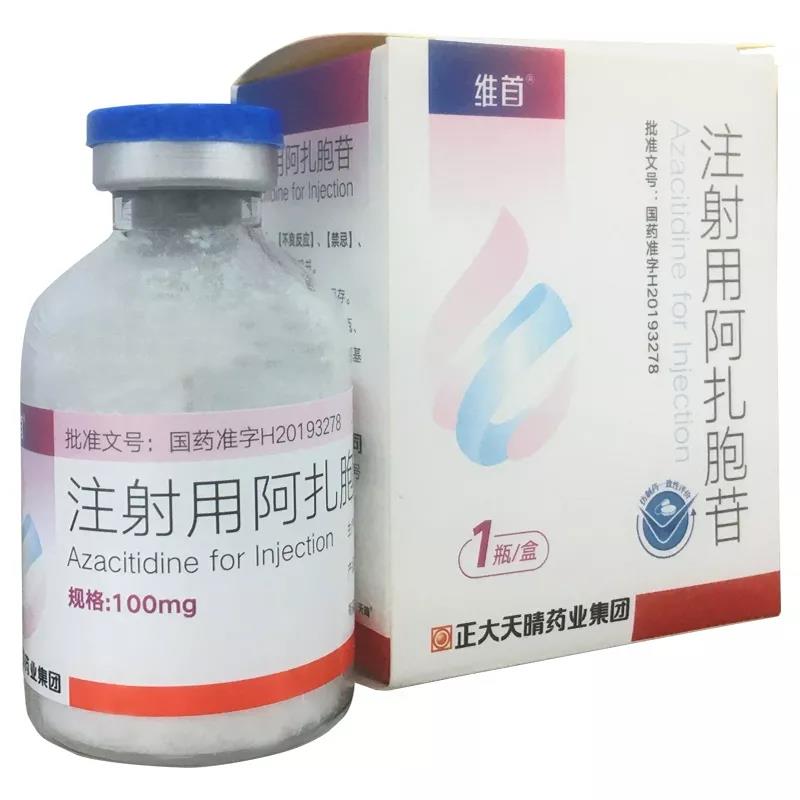
Azacitidine original research
From Celgene (acquired by BMS has been completed, the name Celgene may no longer be available), the domestic equity is in the hands of BeiGene.
Trade name Vidasa, the indication is Myelodysplastic Syndrome (MDS), acute myeloid leukemia (AML) and chronic myelomonocytic leukemia (CMML) with 20-30% of bone marrow blasts. Global sales in 2018 were $ 594 million. Entered the Chinese market in 2017 and was listed in the National Medical Insurance Class B Drugs in October 2018. According to Minnet data, the sales of azacitidine for terminal injection in Chinese public medical institutions in 2018 was 8.48 million yuan, and only the products of original research manufacturers were sold in the domestic market that year.
Azazacitidine is the first MDS treatment approved by the US FDA and is the only demethylation drug that can significantly extend the overall survival of high-risk MDS patients. This product is suitable for the treatment of all subtypes of MDS, and has the qualification to treat rare diseases. For high-risk elderly patients over 65 years of age, azacitidine treatment is the first choice, which can give patients a significant survival advantage.
Pricing
At present, the original retail price of 100mg / branch of Vindasha is 1055 yuan; according to the data of the drug melting circle, the first imitation of Zhengda Tianqing Weishou is currently priced at 996 yuan at 100mg / branch. It is only 59 yuan lower than the original research, and the price drop is 5.6% (the new product listing strategies are different, and the price changes of the two parties in the later period are not ruled out).
Regardless of pricing, in the future, several high-quality generic drugs will work with original research drugs to benefit domestic patients.
The above is the Azazacitidine was first marketed, only 5.6% lower than the original research we have listed for you. You can submit the following form to obtain more industry information we provide for you.
You can visit our website or contact us, and we will provide the latest consultation and solutions
Send Inquiry
Most Popular
lastest New
Send Inquiry

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.